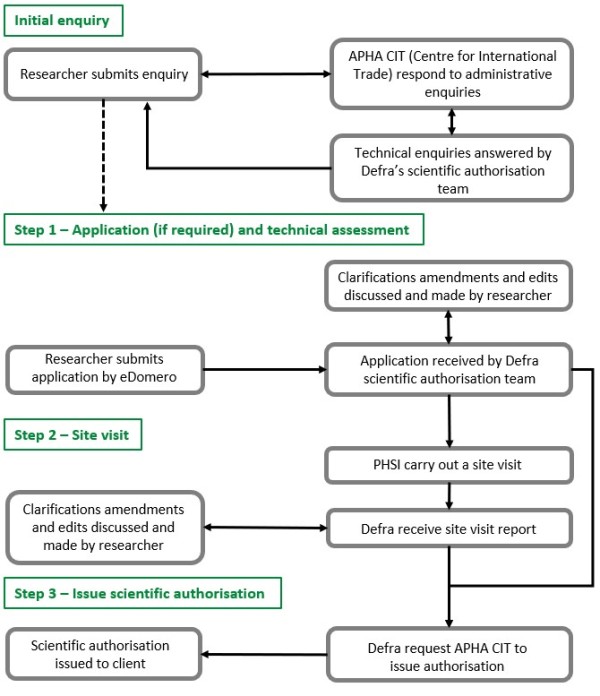
The scientific authorisation process in England and Wales consists of several stages which involve input from Defra, and APHA’s Plant Health and Seed Inspectorate (PHSI) and Centre for International Trade plant health teams (APHA CIT). The process breaks down into three main stages – the technical assessment of the scientific authorisation application (Defra), the site visit (PHSI) and the issuing of the scientific authorisation (APHA CIT). This process is shown in the diagram below. Further information can be found using the following links:
Technical assessment of scientific authorisations
Issuing of scientific authorisations
Further considerations and import requirements not related to Plant Health
Compliance with plant health legislation does not relieve authorisation holders from obligations imposed by other Regulations and requirements and it is their responsibility to ensure that they comply with any other relevant restrictions and requirements. For example, Animal health import licences may be required for invertebrates and there are restrictions and prohibitions on the import of some species of invertebrates which are considered endangered and a prohibition on release of non-native invertebrates. Some other considerations with relevant links are listed below, although this list is not exhaustive:
Licensing of non-native biological control agents
Biopesticides
https://www.hse.gov.uk/pesticides/pesticides-registration/applicant-guide/biopesticides-home.htm
Importing and exporting live animals or animal products
Organisms that could be classed as a fertiliser, soil conditioner, soil improver or biostimulant
These should be referred to the Defra Fertilisers team for further advice -fertilisers@defra.gov.uk.
Convention on International Trade in Endangered Species of Wild Flora & Fauna (CITES)
wildlife.licensing@apha.gov.uk
Genetically Modified Organisms (GMO)
Back to Scientific authorisations guidance for authorisation holders and applicants
